Abstract
Introduction
Relapse remains the most common cause of treatment failure for patients with acute myeloid leukaemia (AML) who undergo allogeneic stem cell transplantation (SCT) and carries a grave prognosis. Multiple studies have identified the presence of minimal residual disease (MRD) prior to SCT assessed by flow cytometry (FCM) as a strong predictor of relapse: however, little is known about the impact of molecular MRD pre-SCT. Molecular techniques can provide 100-1000 fold greater sensitivity than FCM in NPM1 mutated AML allowing identification of patients with very low level MRD prior to SCT. Peri-transplant management decisions for patients with positive molecular MRD are highly challenging due to the absence of robust outcome data.
Methods
Between 2009-14 the NCRI AML17 study enrolled 3215 adult non-APML patients aged 16-77 eligible for intensive chemotherapy. Central screening for NPM1 mutations was positive in 861/2949 (29%); 530 patients provided serial samples for MRD monitoring. Paired blood (PB) and bone marrow aspirates (BM) were requested after each chemotherapy cycle and then quarterly; additional samples were requested pre- and post-SCT. Samples were analysed by RT-qPCR using a suite of 27 mutation-specific reverse primers. Results were only fed back to clinicians after June 2012 when patients could be treated for confirmed re-emergent or persistent molecular positivity. Post-remission treatment was determined according to the validated NCRI risk score, with poor-risk patients recommended for SCT during first complete remission (CR1). Survival analysis was performed using the logrank test.
Results
In total 108/530 patients received SCT (CR1 57 (52%), after molecular relapse or progression (MR) 30 (28%), CR2 21 (19%)). Five-year survival post-SCT (5y-OS) was 65% in CR1, and 54% in MR/CR2 (p=0.3). After MR 26/30 patients received chemotherapy prior to SCT. Evaluable pre-SCT PB and BM samples taken within 60 days of SCT were available for 104 and 78 patients (both available n=74).
Considering PB samples, 5y-OS was 73% (median OS (mOS) not reached (NR)) for MRD-ve patients (n=74) vs. 30% (mOS 8.1 m) for any PB positivity (n=30) (p<0.0001). Patients with a negative pre-SCT BM had 5y-OS of 79% (mOS NR) vs 47% (mOS 11.9 m) if the BM was positive (p=0.002).
Of the 47 patients who received additional chemotherapy for morphologic or molecular relapse or progression, 26 (55%) converted to MRD negativity accounting for 44% of 59 patients who were MRD negative pre-SCT. Ninety-three per cent of 59 pre SCT-MRD negative patients had been MRD negative in the PB after the second cycle of induction (a previously identified marker of favourable outcome).
A threshold of 200 mutant NPM1 transcripts/105 ABL copies in the pre-SCT PB sample split patients into 3 groups with 2y-OS of 81% (negative, n=74), 54% (low, n=13) and 9% (high, n=17; p<0.0001). In the BM a threshold of 1000 copies defined 3 groups with a 2y-OS of 86% (negative, n=36), 56% (low, n=32) and 12.5% (high, n=8; p<0.0001).
Thirty four patients were positive for FLT3-ITD at diagnosis (5y-OS 55%) and 74 were negative (5y-OS 62%; p=0.3). For patients without FLT3 ITD, negative, low and high levels of MRD in the pre-SCT PB sample were associated with 2y-OS of 79% (n=53), 88% (n=8) and 0% (n=9) (p<0.0001). For BM samples, 2y-OS was 78% (n=28), 62% (n=24) and 0% (n=5; p=0.0001). For patients with FLT3 ITD, corresponding 2y-OS for PB samples were 89% (n=21), 0% (n=5) and 25% (n=8; p=0.0005); for BM samples 2y-OS was 83% (n=9), 38% (n=8) and 25% (n=4; p=0.02).
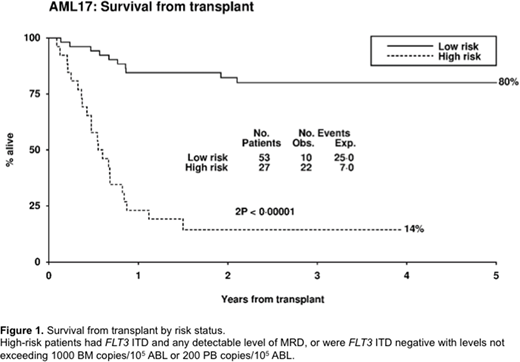
We assigned 80 patients to high and low risk groups based on FLT3 ITD status and pre-SCT MRD level in PB and BM. High-risk patients (FLT3 ITD and any detectable level of MRD in the PB or BM, or FLT3 ITD negative with > 200 copies in the PB or > 1000 copies in the BM) had 3y-OS of 14% (n=27, mOS 6.5 months) compared with 80% for low-risk patients (n=53, p<0.0001) (fig 1).
Conclusions
Patients who test negative for NPM1 mutant transcripts immediately before SCT have a favourable outcome which is also observed in FLT3 ITD negative patients who are MRD positive at levels which do not exceed 200 copies in the PB or 1000 copies in the BM. FLT3 ITD mutated patients with any level of MRD prior to SCT, and FLT3 ITD negative patients with transcript levels above these thresholds, have a very high risk of relapse and may benefit from further chemotherapy or FLT3 inhibition prior to or after SCT; studies to investigate this are urgently needed.
Hills:Daiichi Sankyo: Consultancy, Honoraria. Cavenagh:Celgene: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Takeda: Research Funding, Speakers Bureau; Novartis: Honoraria, Speakers Bureau. Russell:Daiichi Sankyo: Consultancy; Pfizer: Consultancy, Honoraria, Speakers Bureau; Jazz Pharma: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal